plate as a positive control. The assay plates were then incubated (right side up) at
36 to 38°C for another 4 days. A standard prepared from 9mg of griseofulvin in
lmL of DMSO was also assayed for comparison purposes. Positive results were
interpreted as having an inhibition zone between the mycelium growth and the
sample disk as compared to the blank. All positive results were replicated and the
average of the diameters of the zones of inhibited microbial growth was taken.
3. Results and discussion
All inhibition zones of less than 1mm were recorded as negative. In each of the
assays, a standard was included to ensure that the test system was functioning
properly and to compare the activity of the sample of interests with the standard. In
addition, a positive control, which includes the solvent alone, was included, to
measure the effect, if any, of the solvent in which the samples were dissolved.
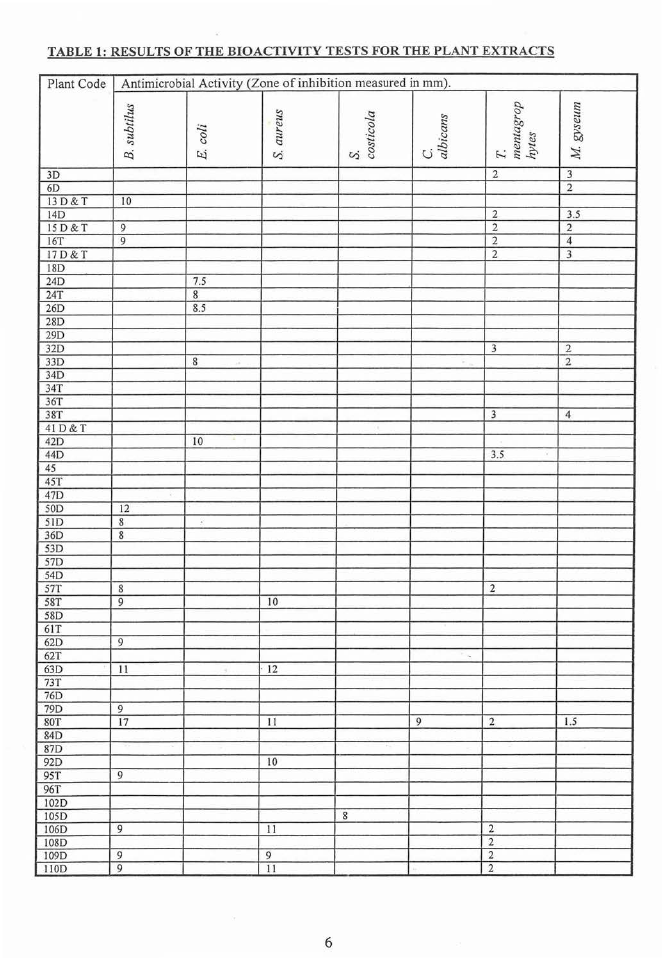
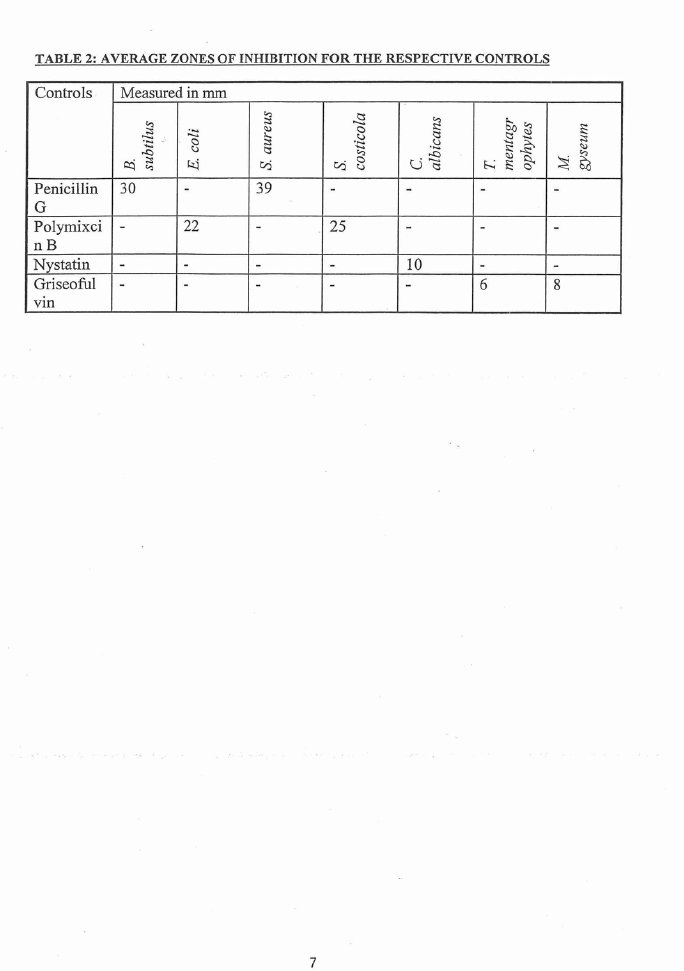
The results from the microbial assays are listed in Table I. The results of the controls
are listed in Table 2.
Approximately half of the extracts showed activity against one of the bacteria and
about a third of them against one of the fungi. Only the Endospermum macrophyllum
wood extract was active against of yeast Candida albicans. This extract was also
active against with three of the bacteria with large zones of inhibition and _both fungi.
Interestingly, the most extensive compilation of medicinal activity of Fijian plants
(Cambie and Ash, 1994) which summarises all written reports of such activity, makes
no mention of this plant. Activity is repo1ied for 450 of the roughly 2,500 plants
found in Fiji. Of course, many traditional treatments have not been documented in
writing and some of the most important ones may be family secrets.
The use of plants can also depend on availability. Many rare plants in Fiji are not
used and some not even named in the local language due to this rareness.
Of the forty-six plants tested eighteen are not listed in Cambie and Ash. Seventeen
are listed but the conditions for which they are reportedly used are not related to anti-
bacterial or antifungal activity. Nine plants were used for anti-bacterial purposes,
Barringtonia asiatica, Vitex trifolia var. subtrisecta, Epipremnum pinnatum, Premna
taitensis, Syzygium malaccense, Solanum torvum, Mussaenda raiateensis, Cordyline
terminalis and Dillenia bi.flora. All of these tested positive for antimicrobial activity,
and all but Barringtonia asiatica for antibacteria activity. The leaves of two plants,
Heritiera littoralis and Xylocarpus granatum, are used to treat thrush, a yeast
intection of the tongue and both displayed strong antifungal activity.
Overall only 44% of plants that did not appear in Cambie and Ash showed any
activity. For plants that were listed as used medicinally but not for antimicrobial uses
53% showed activity. As mentioned earlier, 100% of the 11 plants that were reported
4